Ultraviolet Radiation
Ultraviolet radiation, which is also referred to as UV radiation or ultraviolet rays, is a form of energy traveling through space.
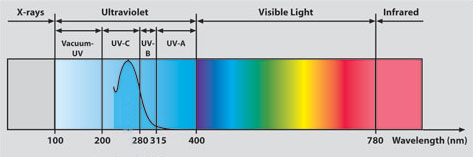 Ultraviolet Radiation and the light-spectrum
Ultraviolet Radiation and the light-spectrumTogether with other forms of energy they are collectively called electromagnetic radiation, and include:
- gamma rays
- infrared rays
- X-rays
- radio waves
UV radiation travels in the form of waves, emanating from the sun, in a wavelength that is invisible to us humans, being shorter than the visible light or infrared wavelengths.
Ultraviolet rays have a wavelength between approximately 100 nanometers and 400 nanometers.
Visible radiation includes wavelengths between 400 and 780 nanometers.
Ultraviolet radiation is further divided into categories according to the wavelengths:
- The shorter ones, UVC and the vacuum UV, are stopped by the earth's atmosphere or ozone layer and so do not reach us here on the earth's surface
- UVB, measuring from 280 - 315 nm, is known as the one to blame for sunburn
- UVA are responsible for deeper penetrating, longer term skin damage, including the dreaded melanoma skin cancer.
Ultraviolet radiation and our skin
Without the ozone protection from the atmosphere, the earth as we know it today would not exist. Once you leave the earth's atmosphere, as with astronauts, the ultraviolet radiation becomes a significant issue.
If the astronaut's skin were to be exposed to this level of ultraviolet radiation, they would be burnt in a matter of seconds.
Even down on planet Earth, protected as we are from some level of UV radiation, it makes sense to be cautious, as the UV rays can cause sunburn as well as permanent damage to your eyes.
How much UV radiation you are exposed to when out and about depends on several factors, including:
- proximity to the equator
- altitude
- time of day
- season of the year
- overcast with cloud cover
If climbing Mount Kilimanjaro on the equator, up to the highest peak, you'd better make sure you are protected as well as possible from the UV rays!
Sunscreens and ultraviolet radiation
Sunscreens help to filter out ultraviolet radiation using a combination of two main types of active ingredients.
- Inorganic particles, such as titanium dioxide or zinc oxide, also called mineral sunscreen ingredients, form a physical barrier on our skin, reflecting or scattering the UV waves.
- Organic sunscreen components, such as oxybenzone and methoxycinnamate, also known as chemical sunscreen ingredients, will absorb the UV rays into your skin and release their energy as heat.
The light-reflecting inorganic compounds used to be known for their opaqueness on the skin, making it look like white paint and only popular for sportsmen such as cricketers and surfers who wore the white cream on their noses and lips.
Today these mineral sunscreen ingredients are micronized into sizes that are no longer visible on the skin's surface.
Containing the same ingredients as before, they are just as effective but far more aesthetically appealing.
Negative Ultraviolet Radiation
 DNA strand can be badly affected by UV radiation
DNA strand can be badly affected by UV radiationThe downside of ultraviolet rays is that they can contain too much energy.
As an example, the energy contained in infrared rays create heat when the molecules of the substance it hits is made to vibrate back and forth.
But because the energy of ultraviolet rays is so much higher, it actually can knock electrons away from the atoms, or even cause molecules to split, instead of just causing the molecules to shake.
So, instead of creating heat, it actually changes the chemical structure of the molecule. For living organisms this can be disastrous, as it can cause cell damage and deformities by actually mutating its genetic code, thus creating a carcinogenic possibility.
Ultraviolet radiation classifications
There are 3 different classifications of ultraviolet rays, based on the amount of energy they contain and their effects on biological matter:
- UVA:
least energetic and least harmful. UVA rays do not have enough energy to break apart the bonds of the ozone, so UVA radiation passes the earth's atmosphere almost unfiltered. - UVB:
higher energy level and shorter wavelength than UVA but lower energy level and a longer wavelength than UVC.
As their energy is often not sufficient to split an ozone molecule, they extend down to the earth's surface. - UVC:
most energetic and most harmful, they don't reach the earth's surface due to being blocked by the ozone layer.
This is because when UVC rays meet the ozone molecules at high layers of the atmosphere, the high energy inherent in them is enough to break apart the bond of the molecule and absorb the energy.
Man-made UVC rays can be very harmful though. eg. in welders.
As both UVB and UVA rays can be detrimental to our health, especially if we overdo our time in the sun, it is important to protect ourselves as best we can.
There are a number of ways that include protective clothing and sunscreens, but also the option of restricting your sun exposure time.
Protection from Ultraviolet Radiation Considerations
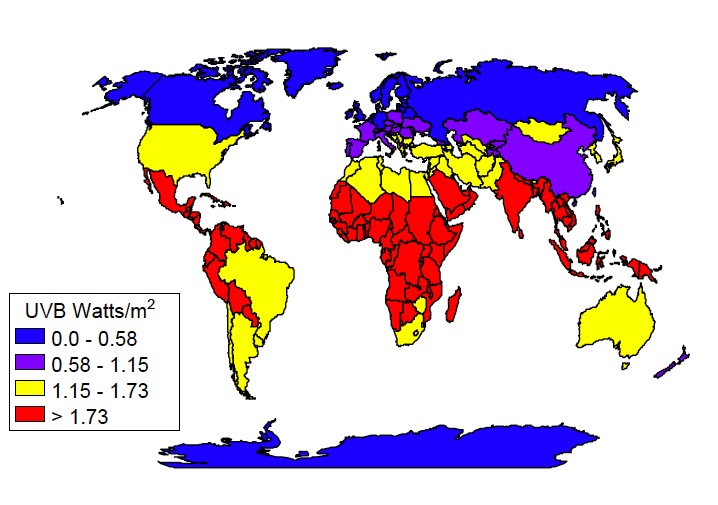 UV radiation varies across the globe
UV radiation varies across the globeThere are a few variables to consider where protection from ultraviolet radiation is concerned:
Position of the sun:
The UV levels will range with the position of the sun in the sky throughout the day as well as the month as in each case the angle of the sun's rays will differ.
At noon, the path through the earth's atmosphere to the earth's surface is the most direct and therefore the shortest and most intense.
This varies throughout the seasons too.
Low ozone coverage:
Without strong ozone protection, the amount of UV that will penetrate is much higher.
Hence the fact that there may be no ozone cover at all is of extreme concern to the amount of UV present in the stratosphere.
Clouds:
Overcast days are confusing as sometimes they protect us and other days they seem to intensify the UV radiation.
This is because clouds can deflect the UV rays back up into space, but in some cases will reflect additional radiation to the ground.
Plus the UVA rays can penetrate clouds, or even glass!
Altitude:
For approximately every 1,000 feet (310m) gained in altitude, you can allow for an increase in UV levels of 4%.
This is due to the air becoming thinner with less molecules able to scatter or absorb the ultraviolet radiation, rather than one being closer to the sun!
Some areas are more protected than others due to the air being thicker due to such things as smog (tropospheric ozone) and dust, soot or sulfates suspended in the air, collectively called aerosols. These all absorb and scatter the UV rays, thus reducing the ultimate ultraviolet radiation levels.
Reflection surfaces:
Different physical surfaces such as snow, water and sand, reflect the UV rays differently but mostly will increase the total ultraviolet radiance levels.
This is due to a phenomenon called albedo whereby some of the ultraviolet rays reflected off the ground encounter scattering by air molecules, aerosols or clouds back down to the earth, increasing the total ultraviolet radiation levels.
Hence, when there is snow on the ground it actually takes a shorter time to get sun burnt than it would on a bright sunny day.
Latitude:
The closer you get to the equator, the higher, or more direct, the sun will be in the sky and the more UV rays you be exposed to. eg. Ultraviolet levels are over 1,000 times higher at the equator than at the polar regions.
In addition, the ozone layer is thinner at the equator as it is over, for example the United States or Europe, so this also contributes to more UV radiation.
Ozone hole
 Penguins affected at the Pole?
Penguins affected at the Pole?Living things and the cells they are made of, are protected from excessive amounts of UV radiation by a colorless gas called ozone.
A layer of ozone in the upper atmosphere absorbs UV radiation and prevents most of it from reaching the Earth.
Yet since the mid-1970s, human activities have been changing the chemistry of the atmosphere in a way that reduces the amount of ozone in the stratosphere (the layer of atmosphere ranging from about 11 to 50 km in altitude).
This means that more ultraviolet radiation can pass through the atmosphere to the Earths surface, particularly at the Poles and nearby regions during certain times of the year.
Without the layer of ozone in the stratosphere to protect us from excessive amounts of UVB and UVA radiation, life as we know it would not exist.
Scientific concern over ozone depletion in the upper atmosphere has prompted extensive efforts to assess the potential damage to life on Earth due to increased levels of UV radiation. Some effects have been studied, but much remains to be learned.
Under the ozone hole in the Polar regions, biologically relevant UV levels are 2-3 times as high as they were before the 1970's when they were officially recognized.
Ultraviolet radiation at different latitudes can be compared through the National Science Foundation (NSF) network in the Antarctica using their satellite ozone data.
There is also an Ozone Hole watch through NASA that allows you to view the latest status of the ozone layer over the Antarctic, with a focus on the ozone hole.
You can get all the ozone facts, some education material, multimedia, and more.




New! Comments
Have your say... please leave me a comment in the box below.